Mitoxantrone is a chemotherapeutic and often used as part of a chemotherapy “cocktail.” Veterinary oncologists often use mitoxantrone for dogs with lymphoma and bladder cancer.
Key Takeaways
- Mitoxantrone is often used in combination with other drugs, and can work quite quickly, depending upon the specifics of the dog’s cancer.
- Mitoxantrone requires very careful handling and is only ever given intravenously at a veterinary hospital.
- Mitoxantrone is sometimes used as a “rescue” drug for dogs who have relapsed.
- The side effects of mitoxantrone for dogs include vomiting, diarrhea, anorexia, and sepsis (secondary to bone marrow suppression). Other serious side effects can occur, including heart toxicity.
- An odd side effect is mitoxantrone can turn the urine and white of the eyes slightly blue for a short time.
What Is Mitoxantrone for Dogs?
Mitoxantrone is a synthetic antineoplastic (anti-cancer drug) in the anthracenedione class of drugs. It is for intravenous (injection into the vein) use only. Veterinarians usually use mitoxantrone for dogs with lymphoma and bladder cancer.
It is similar in action to an older chemotherapeutic, doxorubicin (Adriamycin). Still potent, mitoxantrone is considered less harmful to the heart than doxorubicin.
Brand Names
Novantrone®
How Mitoxantrone for Dogs Works
Mitoxantrone inhibits an enzyme (topoisomerase) that plays a role in opening DNA for repair and alters bonding between DNA resulting in what is called intercalation.
In plain language, mitoxantrone damages cancer cells and prevents them from replicating.
Common Uses of Mitoxantrone for Dogs
Mitoxantrone is often used for bladder tumors (transitional cell carcinomas) and lymphoma. Although not the primary use, mitoxantrone has also been used to treat other cancers.
Bladder Cancer
Treatment of transitional cell carcinoma centers on chemotherapy with mitoxantrone, vinblastine, or carboplatin combined with piroxicam.3 In fact, a 2015 study found that at least 60% of dogs treated with piroxicam combined with either mitoxantrone or carboplatin achieved at least partial response to therapy.1
Mitoxantrone use has also been studied before and after radiation therapy for bladder cancer. In a retrospective study, overall survival was similar; however, it was noted that white blood cell toxicity was more severe in dogs treated with mitoxantrone.4 As such, radiation and mitoxantrone may not be the first-choice option for bladder cancer.
Lymphoma
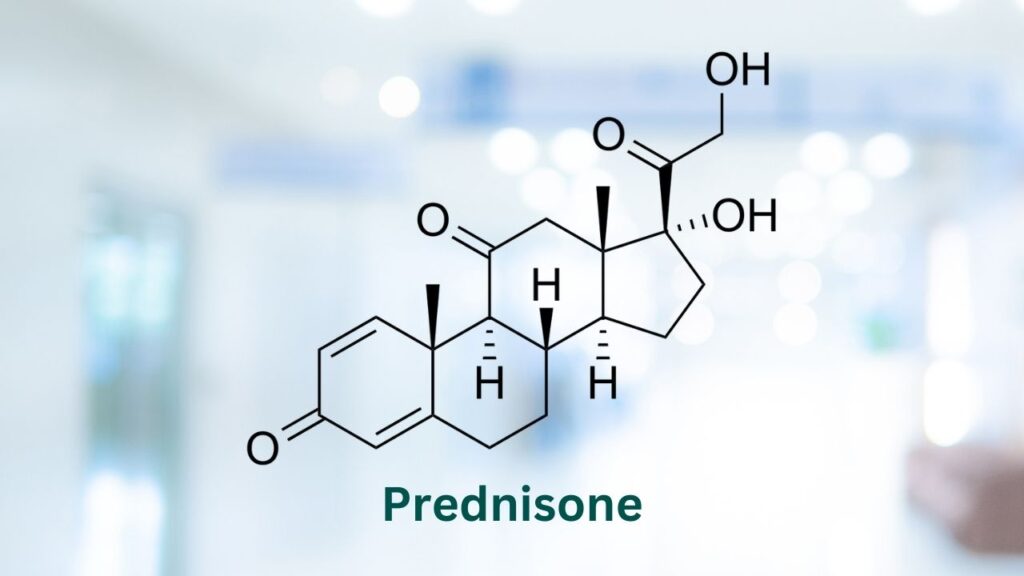
There are many different protocols for the treatment of lymphoma in dogs. Mitoxantrone can be combined with cyclophosphamide, vincristine, and prednisone into a protocol commonly called CMOP.
Two studies found that CMOP protocol provided a mean survival time that was not statistically different and caused fewer side effects than another commonly used protocol, CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone).7,15
This means that the CMOP protocol may be another treatment choice for canine multicentric intermediate- to large-cell lymphoma.15 This may be particularly true for dogs at high risk of developing side effects from the doxorubicin in CHOP.
Mitoxantrone is often used as part of a rescue protocol for lymphoma. (Rescue protocols are used when a dog has either come out of remission or is not responding to the first treatment chosen.)
For example, 14 of 15 dogs who were treated with mitoxantrone and dacarbazine after failing or coming out of remission after L-asparaginase (L)-CHOP responded to this protocol.5 Dogs were more likely to respond to the mitoxantrone and dacarbazine if they had achieved complete remission from the L-CHOP protocol the first time around. In this study, mitoxantrone was administered at 5 mg/m2 IV over 10 min followed by dacarbazine at 600 mg/m2 IV over 5 hr, every 3 wk.
Mesothelioma
For example, dogs diagnosed with malignant mesothelioma have been treated with 5-Fluorouracil (5-FU) only and carboplatin only or alternated with mitoxantrone.9 Mean survival time was eight times longer (234 days) for dogs receiving chemotherapy versus no treatment. This was a small study and additional work is needed to identify the optimal treatment approach for malignant mesothelioma in dogs.
Anal Sac Tumors
In another small study, the medical records from 15 dogs with anal sac adenocarcinoma treated with radiotherapy and mitoxantrone after surgical removal of the primary tumor were reviewed retrospectively.14 The authors felt survival times (median 956 days) were supportive of this type of protocol.
Other Cancers
In another study, ninety-five dogs with various cancers that had already resisted one or more treatment modalities were evaluated.10
- 57 of the dogs had undergone surgery
- 37 got chemotherapy other than mitoxantrone
- 4 had radiation
- 1 was treated with whole-body hyperthermia
Partial or complete remission was obtained in approximately a quarter of all dogs treated with mitoxantrone. Tumors in which there was a partial or complete remission included:10
- Lymphoma
- Squamous cell carcinoma
- Fibrosarcoma
- Thyroid carcinoma
- Transitional cell carcinoma
- Mammary adenocarcinoma
- Hepatocellular carcinoma
- Renal adenocarcinoma
- Rectal carcinoma
- Chondrosarcoma
- Oral malignant melanoma
- Cutaneous malignant melanoma
- Myxosarcoma
- Mesothelioma
- Hemangiopericytoma
When to Not Use Mitoxantrone for Dogs
Your veterinarian may not recommend mitoxantrone due to concerns about cardiac (heart) toxicity. Cardiac toxicity may be more common in patients with prior treatment with anthracyclines (agents with a similar action to mitoxantrone).
A dog treated for lymphoma developed cardiac toxicity after being treated with three different anthracycline class drugs.13 The heart disease was attributed to the medications.
Heart toxicity may also be more common in dogs with prior mediastinal radiotherapy or pre-existing cardiovascular (heart and vessel) disease.
Patients with pre-existing bone marrow suppression generally should not be given mitoxantrone. However, sometimes oncologists decide the benefits to outweigh the risks, so these decisions are always made on a case-by-case basis.
Studies haven’t been done yet to see if mitoxantrone is safe for dogs with pre-existing impaired liver function, so we don’t know if it could be problematic for these dogs.8
How Mitoxantrone Is Given
Mitoxantrone is always given as an intravenous injection in the veterinary office. A common dosage is 5 mg/m2 given once a week, but the oncologist will decide the best dose for each dog’s case.
Mitoxantrone is often combined with cyclophosphamide, vincristine, and prednisone into a protocol often referred to as CMOP. The initial effects of therapy can be seen in the first few weeks.
Mitoxantrone can also be combined with local radiation, but bloodwork needs to be monitored for low white blood cell counts before administration.
What If I Miss a Dose?
Your veterinarian should handle scheduling, so if you need to miss an appointment, speak with your veterinarian.
Storage and Handling
As a chemotherapy drug, mitoxantrone is only handled or given to pets by trained veterinary professionals. The solution is stored at room temperature and must be diluted before use.
Mitoxantrone Side Effects
The most common side effects are:
- Vomiting
- Diarrhea
- Anorexia
- Sepsis secondary to bone marrow suppression
In some cases, other side effects are seen. In one study, overdosing of mitoxantrone was identified immediately after drug administration, with patients needing hospitalization for an average of 8 days. Adverse events recorded in this study included:11
- neutropenia (94%)
- thrombocytopenia (88%)
- anemia (63%)
- diarrhea (63%)
- anorexia (56%)
- vomiting (38%)
- lethargy (31%)
- nausea (25%)
High-grade neutropenia, a specific type of low white blood cells, was incredibly common in the study (94%). The drug filgrastim (brand name Neupogen), which stimulates the growth of white blood cells, was given, but it did not help the neutropenia.
In another study the severity of neutropenia was worse in smaller dogs.12 Overall, dogs weighing less than or equal to 15 kg were more likely to develop the more severe grade 3 or 4 neutropenia. These dogs were often hospitalized.
As previously noted, heart problems are more likely to occur for dogs who have been treated with other anthracycline drugs, have had radiation to the chest, or already have issues with heart function.
Dosage adjustments may need to be made for dogs with impaired liver function, as the liver processes mitoxantrone and its metabolites.2
Blue-Green Pee and Eyes
A very noticeable and startling side effect of mitoxantrone is, thankfully, not dangerous: turning the urine blue-green. The whites of the eyes (sclera) may also turn blue. This is not a sign of overdose or any danger for your dog, and it only lasts for approximately 24 hours after administration.8
- Allstadt, S.D. et al. (2015) “Randomized phase III trial of Piroxicam in combination with mitoxantrone or carboplatin for first-line treatment of urogenital tract transitional cell carcinoma in dogs,” Journal of Veterinary Internal Medicine, 29(1), pp. 261–267. Available at: https://doi.org/10.1111/jvim.12533.
- Batra, V.K. et al. (1986) “Pharmacokinetics of mitoxantrone in man and laboratory animals,” Drug Metabolism Reviews, 17(3-4), pp. 311–329. Available at: https://doi.org/10.3109/03602538608998294.
- Burgess, K. and DeRegis, C. (2019) Urologic oncology, Veterinary Clinics of North America: Small Animal Practice. Elsevier. Available at: https://www.sciencedirect.com/science/article/abs/pii/S0195561618301402?via%3Dihub (Accessed: December 8, 2022).
- Clerc-Renaud, B. et al. (2021) Treatment of genitourinary carcinoma in dogs using nonsteroidal anti-inflammatory drugs, mitoxantrone, and radiation therapy: A retrospective study, Wiley Online Library. Available at: https://onlinelibrary.wiley.com/doi/10.1111/jvim.16078 (Accessed: December 8, 2022).
- Intile, J. et al. (2019) Evaluation of the tolerability of combination chemotherapy with mitoxantrone and dacarbazine in dogs with lymphoma, Allen Press. Allen Press. Available at: https://doi.org/10.5326/JAAHA-MS-6878 (Accessed: December 8, 2022).
- Lawson, H., Musser, M. and Regan, R. (2021) Toxicity, outcome, and management of anthracycline overdoses in 16 dogs. Wiley. Available at: https://onlinelibrary.wiley.com/doi/full/10.1111/jvim.16325 (Accessed: December 9, 2022).
- Marquardt, T. et al. (2019) Substitution of mitoxantrone for doxorubicin in a multidrug chemotherapeutic protocol for first-line treatment of dogs with multicentric intermediate- to large-cell lymphoma, AVMA. American Veterinary Medical Association. Available at: https://doi.org/10.2460/javma.254.2.236 (Accessed: December 8, 2022).
- “Mitoxantrone product insert” (no date). https://www.drugs.com/pro/mitoxantrone.html: https://www.drugs.com/pro/mitoxantrone.html.
- Moberg, H. et al. (2021) Clinical presentation, treatment and outcome of canine malignant mesothelioma: A retrospective study of 34 cases, Online Wiley Library. Available at: https://onlinelibrary.wiley.com/doi/10.1111/vco.12777 (Accessed: December 8, 2022).
- Ogilve, G., Obradovich, J. and Elmslie, R. (1991) “Efficacy of mitoxantrone against various neoplasms in dogs,” J Am Vet Med Assoc, 198(9), pp. 1618–1621.
- Ogilvie, G., Obradovich, J. and Elmslie, R. (1991) “Toxicoses associated with administration of mitoxantrone to dogs with malignant tumors,” J Am Vet Med Assoc, 198(9), pp. 1616–1617.
- Richardson, D. et al. (2018) Correlation between body weight and mitoxantrone-associated neutropenia in dogs, Allen Press. Allen Press. Available at: https://meridian.allenpress.com/jaaha/article-abstract/54/3/144/176470/Correlation-Between-Body-Weight-and-Mitoxantrone?redirectedFrom=fulltext (Accessed: December 9, 2022).
- Tagawa, K., Shimbo, G. and Uemura, A. (2021) Cardiomyopathy in a dog with multicentric lymphoma following treatment with several anthracyclines, Open veterinary journal. U.S. National Library of Medicine. Available at: https://pubmed.ncbi.nlm.nih.gov/33898277/ (Accessed: December 8, 2022).
- Turek, M., Forrest, L. and Ada, W. (2003) Postoperative radiotherapy and mitoxantrone for anal sac adenocarcinoma in the dog: 15 cases (1991-2001). Wiley. Available at: https://onlinelibrary.wiley.com/doi/abs/10.1046/j.1476-5829.2003.00013.x (Accessed: December 8, 2022).
- Wang , S., Lee, J. and Liao, A. (2016) Comparison of efficacy and toxicity of doxorubicin and mitoxantrone in combination chemotherapy for canine lymphoma, The Canadian veterinary journal = La revue veterinaire canadienne. U.S. National Library of Medicine. Available at: https://pubmed.ncbi.nlm.nih.gov/26933263/ (Accessed: December 8, 2022).
Novatrone® is a registered trademark of Immunex Corporation
Topics
Did You Find This Helpful? Share It with Your Pack!
Use the buttons to share what you learned on social media, download a PDF, print this out, or email it to your veterinarian.