Veterinary medicine borrows many medications from human medicine and utilizes research in humans to create studies to validate the drug's use in canine patients. Vinblastine for dogs is one of those drugs that we are fortunate to have applied to our veterinary oncology patients. As knowledge expands in both human and veterinary medicine, we all benefit.
Key Takeaways
- The success rate of vinblastine use in dogs with cancer depends upon the type of cancer being treated.
- Vinblastine, like most chemotherapy, has side effects and we run the risk of those side effects when using the medication.
- Your oncologist will do everything they can to minimize the side effects of vinblastine, which include vomiting, diarrhea, lack of appetite, peripheral nerve damage, decreased white blood cells, lowered immune function, and others.
- Vinblastine for dogs is typically administered every 1-2 weeks.
Vinblastine for Dogs: a Vinca Alkaloid
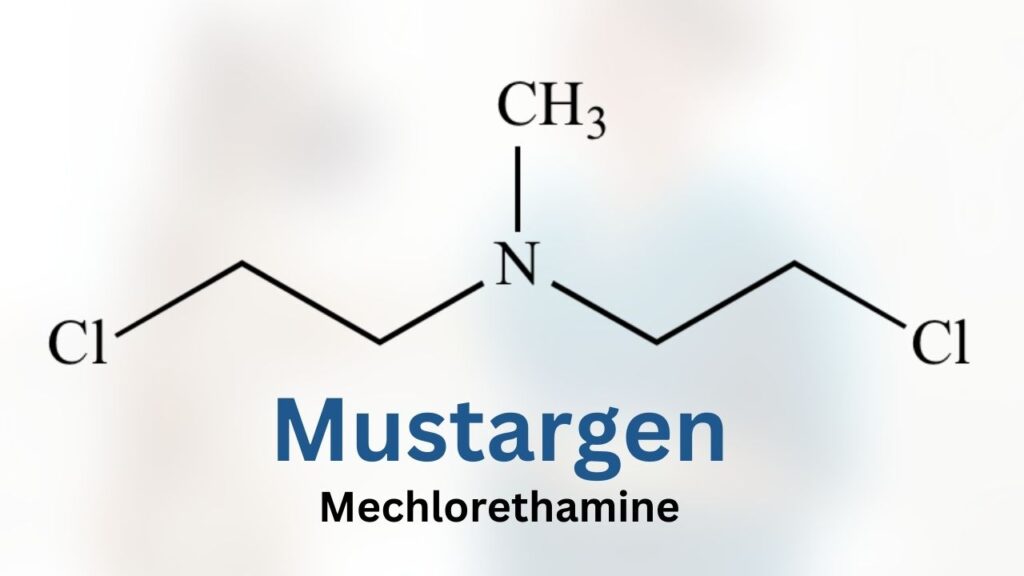
Vinblastine is an injectable anti-cancer drug. It is a human-labeled product, so vinblastine for dogs is technically used off-label (as are most drugs used in veterinary medicine).
Like its cousin vincristine, vinblastine is a vinca alkaloid isolated from the plant Catharanthus roseus (a type of periwinkle)1
How amazing that a chemotherapeutic drug was made from such a pretty garden plant!
Although vincristine and vinblastine are distilled from the same plant, their chemistry, mechanisms of action, and their toxic effects are different. These factors influence which drugs your veterinarian might chose to treat your pet.
Brand Names
Vinblastine is available as a generic medication. However, it is also known as:
29060-LE
NSC-49842
Blastovin®
Cellblastijn®
Cytoblastin®
Fabla®
Lemblastine®
Periblastine®
Serovin®
Solblastin®
Velban®
Velbe®
Velsar®
Xintoprost®
How Vinblastine for Dogs Works
Vinblastine binds to cell proteins and prevents cell division (cell growth), and interferes with cellular amino acid metabolism, disrupting normal cellular function.1
Vinblastine is given intravenously (IV). It becomes heavily bound to proteins in the blood and does not distribute into the central nervous system.
Vinblastine is metabolized in the liver and largely excreted in bile and feces, but it is also excreted in the urine to a lesser extent.1
Common Uses of Vinblastine for Dogs with Cancer
Vinblastine is often used in combination with other drugs. The type and stage of your dog’s cancer, the location of the cancer, and other characteristics all play a role, not only in the choice of using vinblastine, but the success of vinblastine in managing your dog’s disease.
Please note that using vinblastine to treat your dog is based on a discussion between you and your veterinary oncologist. There may be instances when the tumors discussed below may be better treated with other drugs. Or there may be ongoing research that has found other uses for vinblastine that are appropriate for your pet. Be sure to discuss the pros and cons of these choices with your veterinarian.
Lymphoma
Vinblastine typically has fewer side effects than vincristine and can be substituted for vincristine in multidrug lymphoma protocols (eg. CHOP). Vinblastine is not superior to vincristine in its antitumor action. The choice of which to use is a balancing act.
- Higher doses of vinblastine than vincristine are necessary.2
- Veterinarians typically select vinblastine to avoid worsening signs or development of peripheral nerve damage and gastrointestinal upset often caused by vincristine.3
- However, vinblastine can cause greater bone marrow suppression than vincristine.3
Your veterinarian or veterinary oncologist might choose one or the other based on your dog’s medical history or their symptoms as treatment progresses.
Mast Cell Cancer
Vinblastine has been combined with Palladia® (toceranib phosphate) to treat aggressive mast cell tumors.3,4 A 2021 study found this treatment protocol well tolerated but no improvement in tumor control was shown compared to other treatment protocols. Further study is indicated.6
Using vinblastine as a single agent was not as successful as using vinblastine in combination with other medications.7,8 Other medications used to treat mast cell cancer in combination with vinblastine include
- CCNU8
- prednisone9
- cyclophosphamide and prednisone10,11
- prednisone and radiation therapy11
Transitional Cell Carcinoma
In one study, vinblastine was found to have anti-tumor activity for transitional cell carcinoma as a single agent and in combination with the non-steroidal anti-inflammatory drug piroxicam.12
When to Not Use Vinblastine
Vinblastine should not be used, or the dose should be reduced in patients with liver dysfunction, decreased white blood cell counts, or bacterial infections.1
Vinblastine should be used cautiously in dogs with the MDR1 gene mutation (Collies, Australian Sheperd, and other herding breeds may have this mutation).1 Testing may be done before chemotherapy is started to identify the individuals with this mutation.
The following drugs may be contraindicated when your dog is given vinblastine. Let your veterinarian know if your pet is taking these medications, and discuss whether the medication should be administered simultaneously.
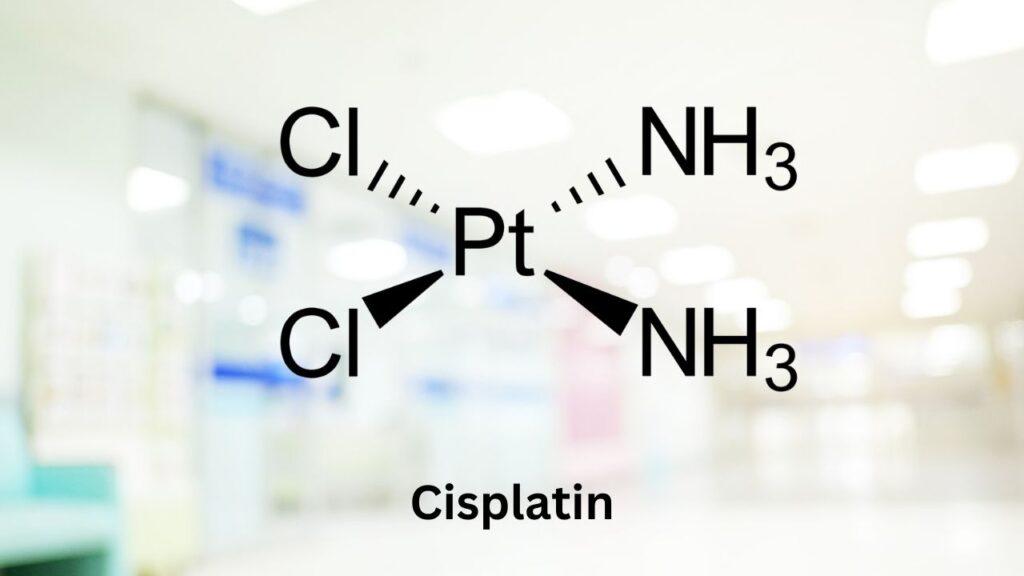
- Ototoxic drugs (drugs that damage hearing), such as cisplatin, and carboplatin
- Immunosuppressive drugs, for example azathioprine, cyclophosphamide, and steroids
- Macrolide antibiotics
- Myelosuppressive drugs (drugs that suppress the bone marrow)
- Vaccines: immunosuppression with vinblastine will diminish the efficacy of vaccines and may increase the likelihood of reactions1
How to Give Vinblastine
Vinblastine is administered intravenously (IV) with the benefit of an intravenous catheter. It is typically administered every 1-2 weeks, often as part of a combined protocol with other drugs.1
What If I Miss a Dose?
Your doctor will let you know how to proceed if a dose is missed.
Storage and Handling
Vinblastine is a strictly intravenous (IV) injectable medication that will not be stored in your home but kept in a veterinary facility and administered by a veterinarian.
Vinblastine should be administered through an IV catheter to prevent potential injection of the medication outside the vein. Injection outside the vein can cause severe tissue irritation.1
Vinblastine Safety
After your dog receives vinblastine, for 3-5 days afterward, the drug may be excreted in urine, feces or vomit. Wear chemotherapy-resistant gloves when cleaning and place everything in sealed plastic bags and dispose of the materials.1 Persons pregnant, nursing or attempting to conceive should not clean the pet’s waste.1 Precautions should be taken when handling dog toys, food and water bowls.1
Vinblastine Side Effects
Decreased appetite, vomiting, and diarrhea are possible side effects of most chemotherapy drugs.
To help with side effects, your veterinarian can prescribe supportive medications to make your pet feel better. If side effects are persistent or severe, they should be reported to your veterinarian.1
Difficulty breathing, neurologic signs, irritation or skin sloughing at the injection site are rare side effects and should be reported immediately to your veterinarian.1
Since Vinblastine suppresses the rapidly dividing cells in the bone marrow, it can cause an increased chance of infection.1 Persistent lethargy, fever, inappetence (your dog not eating), or any other changes in demeanor should be reported to your veterinarian.
Dog lovers sometimes worry about using potent drugs like vinblastine. While this fear is normal, consider that veterinary oncologists always aim to give doses low enough to keep side effects extremely low and, if possible, nonexistent.
Monitoring
A baseline CBC (complete blood count) should be done before every treatment to ensure that your pet has enough white blood cells and platelets to safely treat. Monitoring liver enzymes periodically is a good idea as well.1
These tests will help your dog avoid side effects due to continued dosing.
- Plumb DC. Vinblastine. Plumb’s Veterinary Drugs. https://app.plumbs.com/drug-monograph/vTu5mODx1yPROD. Updated September 2022 Accessed December 2022.
- Harding K, Bergman N, Smith A , et al. Response rate to a single dose of vinblastine administered to dogs with treatment-naive multicentric lymphoma. Vet Comp Oncol. 2018; 16: 636– 641. https://doi.org/10.1111/vco.12433
- Mullin C, Clifford C. Which drugs are used for the medical management of Lymphoma in Dogs & Cats? Plumbs Therapeutic Briefs. Nov 2016. Jan 2023. htps://files.brief.vet/migration/article/32126/rx_which-drugs-are-used-for-medical-management-of-lymphoma-in-dogs–cats-32126-article.pdf
- Robat C, London C, Bunting L, McCartan L, et al. Safety evaluation of combination vinblastine and toceranib phosphate (Palladia®) in dogs: a phase I dose-finding study. Vet Comp Oncol. 2012 Sep;10(3):174-83. doi: 10.1111/j.1476-5829.2011.00261.x. epub 2011 Jan 31. PMID: 22235914; PMCID: PMC3837095.
- Kim J, Yi H, Song K, Seo Kyoung. Secondary lymphoma development after chemotherapy in three dogs. Veterinary Medicine and Science. 7(4): 1144-1149. https://doi.org/10.1002/vms3.474
- Todd J, Nguyen S, White J, Langova V, et al. Combination vinblastine and palladia for high-grade and metastatic mast cell tumors in dogs. Can Vet J. 2021 Dec;62(12):1335-1340. PMID: 34857971; PMCID: PMC8591577.
- Rassnick K, Bailey D, Flory A, Balkman C, Kiselow M, et al. (2008), Efficacy of Vinblastine for Treatment of Canine Mast Cell Tumors. Journal of Veterinary Internal Medicine, 22: 1390-1396. https://doi.org/10.1111/j.1939-1676.2008.0195.x
- Cooper M, Tsai X and Bennett P. (2009), Combination CCNU and vinblastine chemotherapy for canine mast cell tumours: 57 cases. Veterinary and Comparative Oncology, 7: 196-206. https://doi.org/10.1111/j.1476-5829.2009.00190.x
- Webster J, Yuzbasiyan-Gurkan V, Thamm D. et al. Evaluation of prognostic markers for canine mast cell tumors treated with vinblastine and prednisone. BMC Vet Res 4, 32 (2008). https://doi.org/10.1186/1746-6148-4-32
- Camps-Palau M, Leibman N, Elmslie R, et al. (2007), Treatment of canine mast cell tumours with vinblastine, cyclophosphamide and prednisone: 35 cases (1997–2004). Veterinary and Comparative Oncology, 5: 156-167. https://doi.org/10.1111/j.1476-5829.2006.00125.x
- Camps-Palau, M.A., Leibman, N.F., Elmslie, R., Lana, S.E., Plaza, S., McKnight, J.A., Risbon, R. and Bergman, P.J. (2007), Treatment of canine mast cell tumours with vinblastine, cyclophosphamide and prednisone: 35 cases (1997–2004). Veterinary and Comparative Oncology, 5: 156-167. https://doi.org/10.1111/j.1476-5829.2006.00125.x
- Arnold, E, Childress M, Fourez L, et al. (2011), Clinical Trial of Vinblastine in Dogs with Transitional Cell Carcinoma of the Urinary Bladder. J Vet Intern Med, 25: 1385-1390. https://doi.org/10.1111/j.1939-1676.2011.00796.x
NSC-49842® is a registered trademark of Selleck Chemicals
Blastovin® is a registered trademark of Teva
Cellblastin® is a registered trademark of Cell Pharm
Cytoblastin® is a registered trademark of Cipla Ltd.
Lemblastine® is a registered trademark of Teva Chile Laboratorio
Periblastine® is a registered trademark of Intramed
Serovin® is a registered trademark of Jaywin Remedies Pvt Ltd
Solblastin® is a registered trademark of Faulding
Velban® is a registered trademark of Eli Lilly
Velbe® is a registered trademark of Genus Pharmaceuticals Limited
Velsar® is a registered trademark of Pfizer
Xintoprost® is a registered trademark of Richmond Laboratorios
Topics
Did You Find This Helpful? Share It with Your Pack!
Use the buttons to share what you learned on social media, download a PDF, print this out, or email it to your veterinarian.